Rukmava made it to Image of Research Finalists!!!
Congratulations to the 12 graduate students who placed in this year's exhibit! Heading link
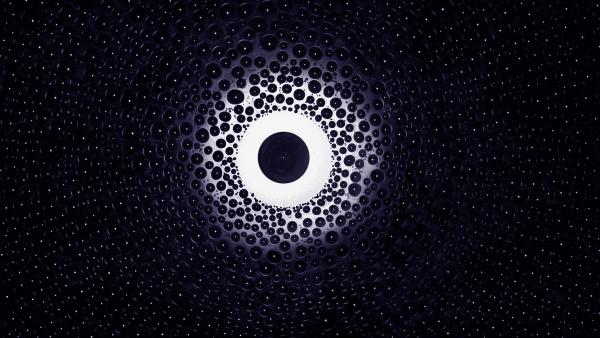
Come winter and the environment is draped in a snowy blanket, immensely diverse yet infinitely monotonous. But there’s more to this icy monochromatic beauty, with the ubiquitous menace of icing engendering slippery roads and bald tires. Rock salts are the common choice of chemical deicers, used to steer clear of icy sidewalks and roadways. These salts work by lowering the freezing point of water, precluding ice formation. And it is this working mechanism of salt which the current image (captured as a part of my ongoing doctoral research on anti-icing systems) intends to convey. To demonstrate this, I carried out an experiment with a single sodium chloride salt crystal subjecting it to an extremely humid and frigid atmosphere; conditions favorable for icing. The central salty drop remained unfrozen, untouched by virtue of an annular region of inhibited condensation which occurs due to a variation of water vapor concentration above the salty drop and neighboring population of supercooled water droplets. And this is how, salts prevent ice formation for extended periods of time.