AnandLab students are winners at UIC’s image of research competition
Introduction
This year three AnandLab students were the winners (1st and 2nd)+finalists of UIC's image of research competition...
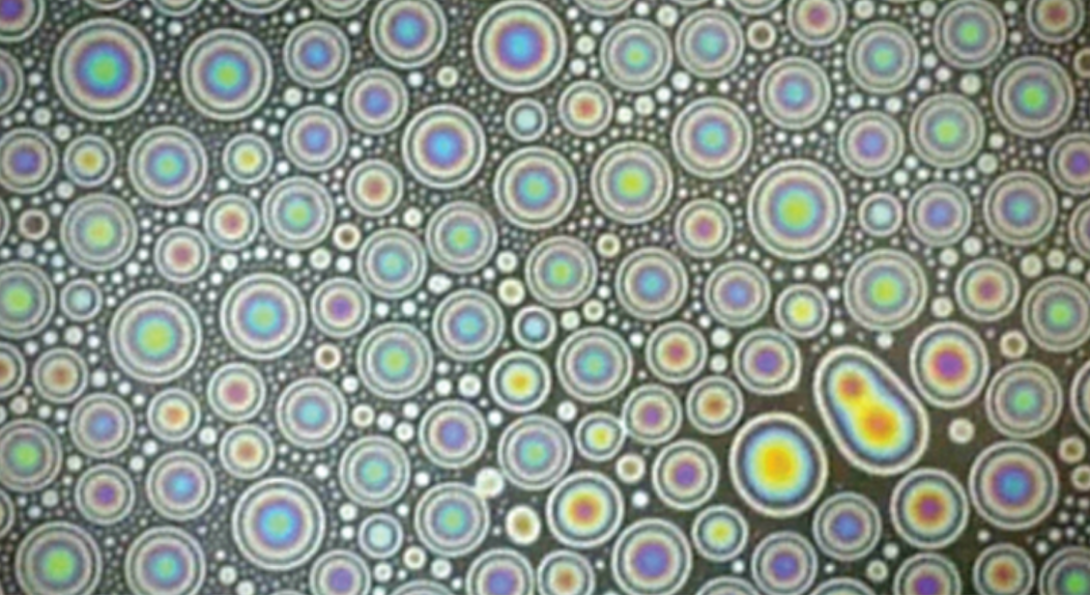
Mechanical engineering student Hassan Bararnia won first place in the still images category with “Oil Marbles on Water Interface,” which demonstrates how tiny oil droplets float and merge at the water-air interface.
Congratulations to Navid for winning the 2nd Place in 'Moving Image Category'
Congratulations to Rukmava for being the finalist with 'Winter 'Fail''
Oil Marbles on Water Interface
Submitted by: Hassan Bararnia
Program: Mechanical Engineering
Division: Engineering, Mathematics, and Physical Sciences
Description: Rainbow-type interference is a ubiquitous part of our daily life, explaining colorful oil spillage or a sunlit soap bubble. When light strikes a thin surface with thickness smaller than the wavelength of light, the reflected light waves from upper and lower boundaries of the surface can reinforce each other, giving rise to an intensified color depending on the surface’s thickness. A thin aqueous film with a varied thickness or curvature-like bubble can lead to the formation of colorful patterns. Based on the same mechanism, an oil spill on water exhibits fringes due to its uneven thickness and different refractive indexes compared to water and air. In recent years, a large number of studies have been focused on removing oil spills due to their damage to the ecosystem. Having detailed information about the evolution and dynamics of oil droplets at the water-air interface by the presence of active molecules enables experts to propose better strategies to these efforts. The presence of surface-active agents promote the spreading of oil and increase the frequency of coalescence events between them, leading to faster formation of oil films. The current snapshot demonstrates how tiny oil droplets float and merge at the water-air interface.
Winter ‘Fail’
Submitted by: Rukmava Chatterjee
Program: Mechanical Engineering
Division: Engineering, Mathematics, and Physical Sciences
Description: Ice crystal growth represent one of the finest examples of symmetry in nature amidst the chaos that encompasses it’s genesis. The birth of ice crystals is a song of ambient temperature and water vapor supersaturation with their growth being dictated by a marriage of non-equilibrium molecular dynamics at the ice/water and ice/vapor interfaces engendering a rich phenomenology of solidification behaviors under different environs. To elucidate the mechanism of ice crystal’s pattern formation and intertwined morphological instability, we have examined the growth of a single crystal from water vapor, focusing on the underlying physical processes that govern their growth rates and structure formation. This is what the current image (captured as a part of my ongoing doctoral research at UIC) intends to convey. To demonstrate the hypnotic beauty of this rare jewel amidst an unkind wintry wind, I have carried out experiments in a simulated cloud chamber under controlled frigid environmental conditions. The fundamental investigation of the life cycle of ice crystal will advance our understanding of the efficacy of novel materials in preventing ice/frost formation on functional surfaces, bringing us a step closer in defeating nature’s artist at its own craft.
Calm before the Storm
Calm Before The Storm
Submitted by: Navid Saneie
Program: Mechanical Engineering
Division: Engineering, Mathematics, and Physical Sciences
Description: When a liquid drop comes close to a hot surface with a temperature above the liquids’ boiling point, we expect to observe a behavior that resembles the liquid boiling. However, if the surface temperature is significantly higher than the boiling point, the evaporation rate of the liquid drop becomes high enough that it creates a vapor cushion below the droplet preventing any contact between the liquid and the substrate. This phenomenon, known as Leidenfrost effect, represents a very low rate of heat transfer from the hot surface. During my research at UIC, I’ve studied the behavior of individual droplets after contacting superheated microstructured surfaces. With the goal of understanding and recognizing the dominant heat transfer mechanisms during the droplet impact, I’ve fabricated microstructures to systematically study the boiling transitions inside an impacting liquid droplet. This video is explaining a very interesting incident at which the droplet shows an explosion behavior after it contacts a substrate with a dense pattern of fabricated microstructures. During this explosion, even though the droplet shows a similar behavior to when it boils, boiling is suppressed, and the explosion is not a sign of efficient heat transfer. Recognizing this behavior is vital to avoid any possible calamities in power plants and industries in which cooling hot surfaces is critical.