Our work on Fundamentals of impure ice adhesion is now online at Materials Horizons !!! Featured in UIC News!!!
Big congratulations!!!
Pleased to share our latest work on the fundamentals of ice adhesion appearing in the Royal Society of Chemistry's journal 𝑴𝒂𝒕𝒆𝒓𝒊𝒂𝒍𝒔 𝑯𝒐𝒓𝒊𝒛𝒐𝒏𝒔 authored by my fantastic student and AnandLab alumnus Rukmava Chatterjee.
Thanks to UIC News for the cover story on our work!
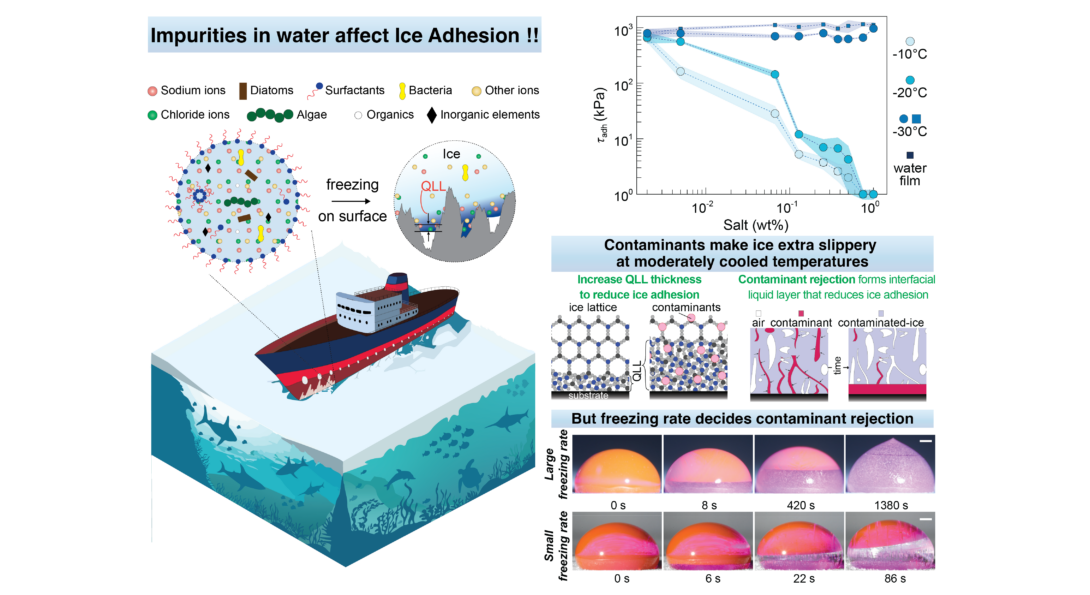
For decades, scientists have been trying to figure out coatings that can make ice less sticky. But everyone typically uses ice formed by freezing pristine water to quantify how well ice sticks to surfaces. In reality, water is seldom pure! Here we take a deep dive on how those impurities affect how well ice sticks to the surface...
Results: Impurities like salt, solvents, and soap molecules make ice extra slippery at moderate temperatures. But at deeply cooled conditions, salt makes ice adhesion stronger!
This work has been years in the making. Want to give a shout-out to AnandLab alumnus Rajith Unnikrishnan for laying the groundwork of this research, to my fabulous student Christopher Carducci who worked tirelessly to get MicroComputer Tomography of Ice, to AnandLab alumnus Vijay Prithiv Bathey for working on contaminant rejection. Thanks to my dear friend and collaborator Subramanian Sankaranarayanan and his group members (Suvo Banik, Arnab Neogi, Suman Chakraborty) we also got some deep insights into the nature of QLL and how it changes with impurities. Finally, big thanks to NSF and DOE for supporting this work...
Check the attached infographic and to find out more: Read the article here.